What Causes Gas Pressure In A Container Such As A Helium Balloon
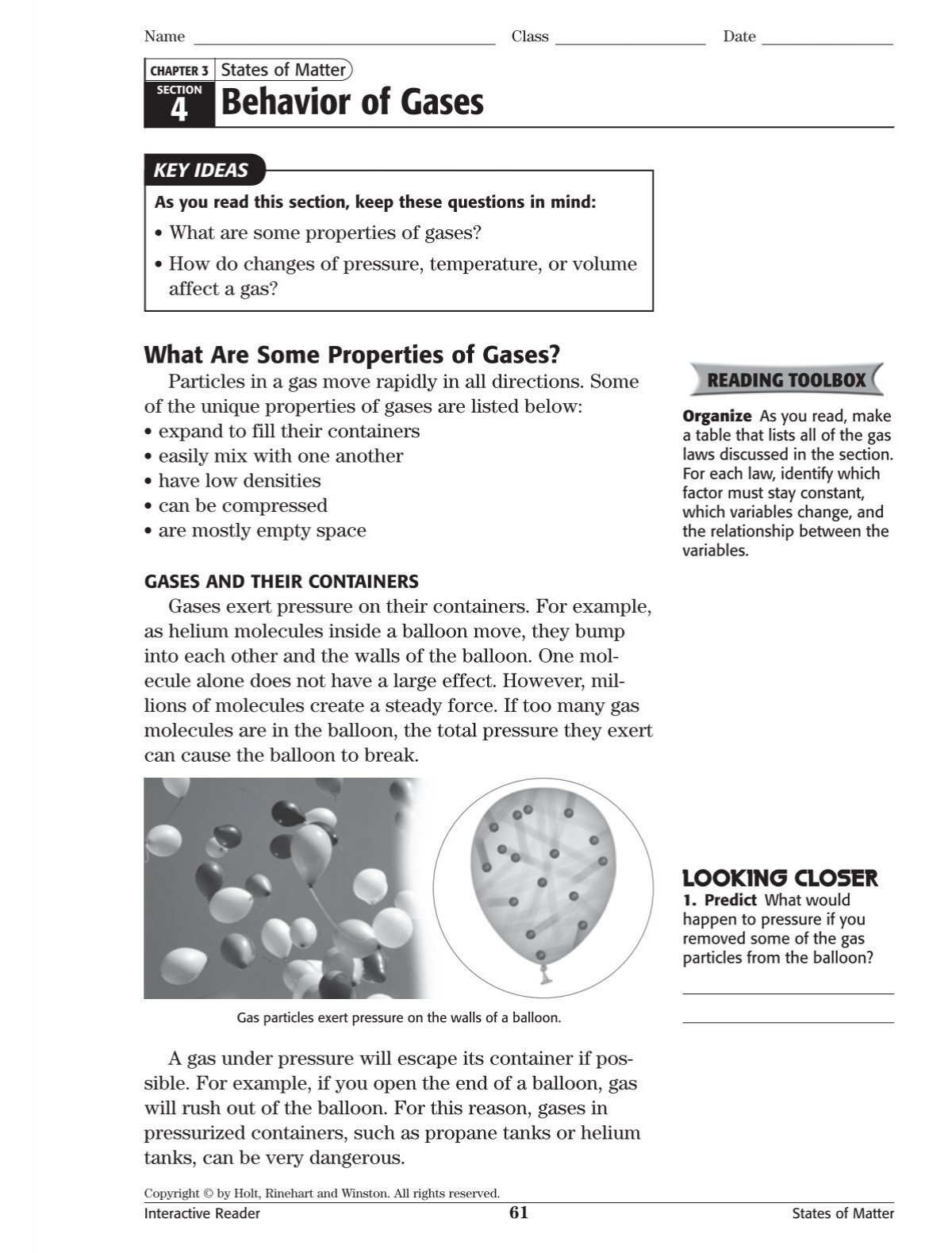
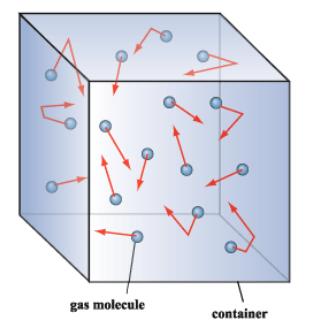
What causes gas pressure in a container such as a helium balloon. The force acting on the container due to these collisions is at right angles to the container. A more molecules have enough energy to overcome the attractive forces holding. The simultaneous collisions of fast-moving particles in the container d.
Because of the fact the balloon rises the. What causes gas pressure in a container such as a helium balloon. The container isnt finished.
The simultaneous collisions of fast-moving particles with the container. Uses advised against Industrial or technical grade unsuitable for medical applications or inhalation. The simultaneous collisions of fast-moving particles with the container d.
With which temperature scale is temperature directly proportional to. What causes gas pressure in a container such as a helium balloon. What causes gas pressure in a container such as a helium balloon.
D particles with the highest kinetic energy Why does a liquids rate of evaporation increase when the liquid is heated. The simultaneous collisions of fast-moving particles in the container d. What cause gas pressure in a container such as a helium balloon.
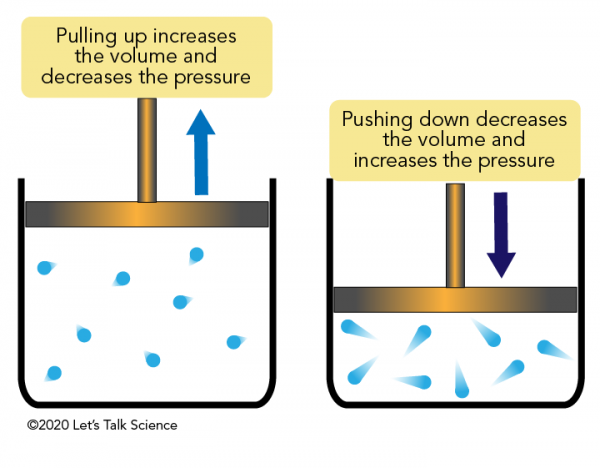
For example the collisions caused by a gas trapped inside a balloon cause forces to act outwards in. If the balloon is flexible then it expands as it rises to altitudes with lower air pressure. An example of this is when a gas is trapped in a cylinder by a piston.
The vacuum maintained in the container c. It is the collisions of the air molecules with the inside walls of the balloon that keep the balloon inflated.
The container isnt finished.
What is the key difference between a liquid and a gas. D particles with the highest kinetic energy Why does a liquids rate of evaporation increase when the liquid is heated. The more often the particles hit the walls and the faster they are moving when they do this the higher the pressure. With which temperature scale is temperature directly proportional to. Uses advised against Industrial or technical grade unsuitable for medical applications or inhalation. If the piston is pushed in the gas particles will have less. An example of this is when a gas is trapped in a cylinder by a piston. The container isnt finished. What is the key difference between a liquid and a gas.
13 Details of the supplier of the safety data sheet Supplier. The force acting on the container due to these collisions is at right angles to the container. What causes gas pressure in a container such as a helium balloon. C the simultaneous collisions of fast-moing particle with the container Which are the first particles to evaporate from a liquid. Atmospheric pressure acting on the outside walls of the container. Of course the outside atmosphere similarly exerts a pressure on the outside of the balloon. Certainly the stress interior a huge helium balloons used for climate monitoring is atmospheric.


























Post a Comment for "What Causes Gas Pressure In A Container Such As A Helium Balloon"